The advent of portable electronics and renewable energy sources with intermittent production has significantly increased the demand for safe, high-energy density, and high-power energy storage materials. In the Rowan group, we are applying our broad expertise in functional polymeric materials to solve challenges ranging from redox-flow batteries for grid storage to solid-state lithium-ion batteries for portable electronics.
Current Projects
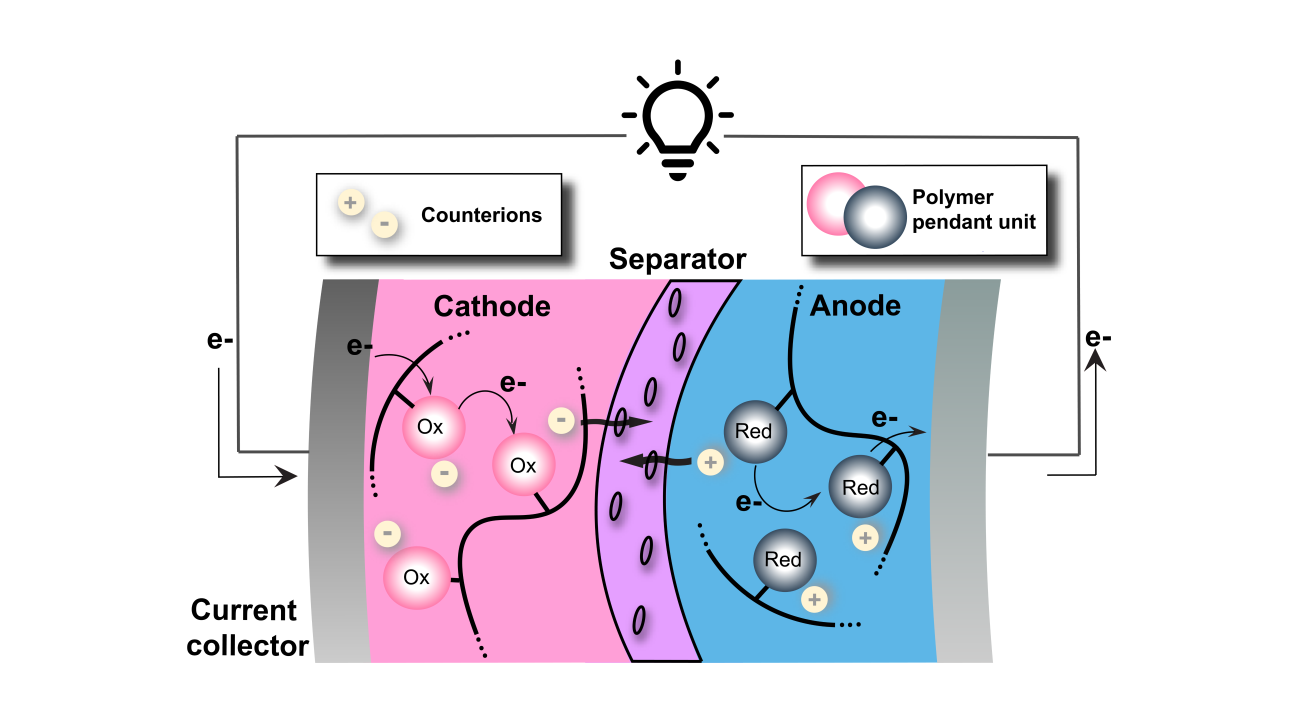
Redox-active insoluble particles are particularly intriguing as redox-active materials in redox flow batteries (RFB) owing to their size and shape, which prevent membrane crossover by size exclusion mechanism while simultaneously reducing electrolyte viscosity compared to soluble redox-active materials. We are focusing on synthesizing novel redox-active particles, investigating the morphological properties, and characterizing the corresponding influence on battery performance.
Current lithium battery technologies have revolutionized portable electronics, but continue to suffer from safety concerns as dendrite growth on the anode surface can lead to shorting and overheating that limit potential applications. One of the most promising solutions to this issue is to replace the flammable liquid electrolyte with a solid polymer electrolyte that can conduct ions while also suppressing dendrite growth. Many traditional ion-conducting polymers suffer from poor adhesion to the rough, porous electrode surface which leads to poor cycle life and failure. We are applying our expertise in structurally dynamic materials to develop reprocessable networks capable of stimuli-activated adhesion and ion conduction. Dynamic, ion-conducting networks could then be used as a component in replacement electrolytes that accommodate a range of uses from micro-grid storage to manned deep-space exploration.
Redox-active polymers for battery electrodes serve as a pathway to more sustainable energy storage through organic batteries. Our approach is to use the intrinsic tunability of polymer materials to design high performance battery electrodes in a highly collaborative effort that takes advantage of materials engineering, electrochemistry, and machine learning.
Selected Publications
Abstract:
Cross-linked polymer electrolytes containing structurally dynamic disulfide bonds have been synthesized to investigate their combined ion transport and adhesive properties. Dynamic network polymers of varying cross-link densities are synthesized via thiol oxidation of a bisthiol monomer, 2,2′-(ethylenedioxy)diethanethiol, and tetrathiol cross-linker, pentaerythritol tetrakis(3-mercaptopropionate). At optimal loading of lithium bis(trifluoromethane-sulfonyl-imide) (LiTFSI) salt, the ionic conductivities (σ) at 90 °C are about 1 × 10–4 and 1 × 10–5 S/cm at the lowest and highest cross-linking, respectively. Notably, in comparison to the equivalent nondynamic network, the dynamic network shows a positive deviation in σ above 90 °C, which suggests the enhancement of ion transport occurs from the difference in structural relaxation on account of the dissociation of disulfide bonds. Lap shear adhesion and conductivity tests on ITO-coated glass substrates reveal the dynamic network exhibits a higher adhesive shear strength of 0.2 MPa (vs 0.03 MPa for the nondynamic network) and higher σ after the application of external stimulus (UV light or heat). The adhesive strength and σ are stable over multiple debonding/rebonding cycles and, thus, demonstrating the utility of these structurally dynamic networks as solid polymer electrolyte adhesives.

Abstract:
Cross-linking poly(glycidyl methacrylate) microparticles with redox-responsive bis(5-amino-l,3,4-thiadiazol-2-yl) disulfide moieties yield redox-active particles (RAPs) capable of electrochemical energy storage via a reversible 2-electron reduction of the disulfide bond. The resulting RAPs show improved electrochemical reversibility compared to a small-molecule disulfide analogue in solution, attributed to spatial confinement of the polymer-grafted disulfides in the particle. Galvanostatic cycling was used to investigate the impact of electrolyte selection on stability and specific capacity. A dimethyl sulfoxide/magnesium triflate electrolyte was ultimately selected for its favorable electrochemical reversibility and specific capacity. Additionally, the specific capacity showed a strong dependence on particle size where smaller particles yielded higher specific capacity. Overall, these experiments offer a promising direction in designing synthetically facile and electrochemically stable materials for organosulfur-based multielectron energy storage coupled with beyond Li ion systems such as Mg.
